coal
Coal (Old English col; meaning “mineral of fossilized carbon” since the thirteenth century) is a combustible black or brownish-black sedimentary rock usually occurring in rock strata in layers or veins called coal beds or coal seams. The harder forms, such as anthracite coal, can be regarded as metamorphic rock because of later exposure to elevated temperature and pressure. Coal is composed primarily of carbon along with variable quantities of other elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.[1] A fossil fuel, coal forms when dead plant matter is converted into peat, which in turn is converted into lignite, then sub-bituminous coal, after that bituminous coal, and lastly anthracite. This involves biological and geological processes that take place over a long period.
Throughout history, coal has been used as an energy resource, primarily burned for the production of electricity and/or heat, and is also used for industrial purposes, such as refining metals. Coal is the largest source of energy for the generation of electricity worldwide, as well as one of the largest worldwide anthropogenic sources of carbon dioxide releases. The extraction of coal, its use in energy production and its byproducts are all associated with environmental and health effects including Climate change.[2]
Coal is extracted from the ground by coal mining. Since 1983, the world’s top coal producer has been China.[3] In 2011 China produced 3,520 million tonnes of coal – 49.5% of 7,695 million tonnes world coal production. In 2011 other large producers were United States (993 million tonnes), India (589), European Union (576) and Australia (416).[3] In 2010 the largest exporters were Australia with 328 million tonnes (27.1% of world coal export) and Indonesia with 316 million tonnes (26.1%),[4] while the largest importers were Japan with 207 million tonnes (17.5% of world coal import), China with 195 million tonnes (16.6%) and South Korea with 126 million tonnes (10.7%).[5]
Etymology
The word originally took the form col in Old English, from Proto-Germanic *kula(n), which in turn is hypothesized to come from the Proto-Indo-European root *g(e)u-lo- “live coal”.[6] Germanic cognates include the Old Frisian kole, Middle Dutch cole, Dutch kool, Old High German chol, German Kohle and Old Norse kol, and the Irish word gual is also a cognate via the Indo-European root.[6] In Old Turkic languages, kül is “ash(es), cinders”, öčür is “quench”. The compound “charcoal” in Turkic is öčür(ülmüş) kül, literally “quenched ashes, cinders, coals” with elided anlaut ö- and inflection affixes -ülmüş.[7]
The word took on the meaning “mineral consisting of fossilized carbon” in the thirteenth century.[6]
Formation
At various times in the geologic past, the Earth had dense forests in low-lying wetland areas. Due to natural processes such as flooding, these forests were buried underneath soil. As more and more soil deposited over them, they were compressed. The temperature also rose as they sank deeper and deeper. As the process continued the plant matter was protected from biodegradation and oxidation, usually by mud or acidic water. This trapped the carbon in immense peat bogs that were eventually covered and deeply buried by sediments. Under high pressure and high temperature, dead vegetation was slowly converted to coal. As coal contains mainly carbon, the conversion of dead vegetation into coal is called carbonization.[8]
The wide, shallow seas of the Carboniferous Period provided ideal conditions for coal formation, although coal is known from most geological periods. The exception is the coal gap in the Permian–Triassic extinction event, where coal is rare. Coal is known from Precambrian strata, which predate land plants — this coal is presumed to have originated from residues of algae.[9][10]
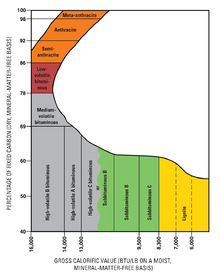
Ranks
As geological processes apply pressure to dead biotic material over time, under suitable conditions, its metamorphic grade increases successively into:
- Peat, considered to be a precursor of coal, has industrial importance as a fuel in some regions, for example, Ireland and Finland. In its dehydrated form, peat is a highly effective absorbent for fuel and oil spills on land and water. It is also used as a conditioner for soil to make it more able to retain and slowly release water.
- Lignite, or brown coal, is the lowest rank of coal and used almost exclusively as fuel for electric power generation. Jet, a compact form of lignite, is sometimes polished and has been used as an ornamental stone since theUpper Palaeolithic.
- Sub-bituminous coal, whose properties range from those of lignite to those of bituminous coal, is used primarily as fuel for steam-electric power generation and is an important source of light aromatic hydrocarbons for thechemical synthesis industry.
- Bituminous coal is a dense sedimentary rock, usually black, but sometimes dark brown, often with well-defined bands of bright and dull material; it is used primarily as fuel in steam-electric power generation, with substantial quantities used for heat and power applications in manufacturing and to make coke.
- “Steam coal” is a grade between bituminous coal and anthracite, once widely used as a fuel for steam locomotives. In this specialized use, it is sometimes known as “sea coal” in the US.[11] Small steam coal (dry small steam nuts or DSSN) was used as a fuel for domestic water heating.
- Anthracite, the highest rank of coal, is a harder, glossy black coal used primarily for residential and commercial space heating. It may be divided further into metamorphically altered bituminous coal and “petrified oil”, as from the deposits in Pennsylvania.
- Graphite is one of the more difficult coals to ignite and is not commonly used as fuel — it is mostly used in pencils, and when powdered, as a lubricant.
The classification of coal is generally based on the content of volatiles. However, the exact classification varies between countries. According to the German classification, coal is classified as follows:[12]
| German Classification | English Designation | Volatiles % | C Carbon % | H Hydrogen % | O Oxygen % | S Sulfur % | Heat content kJ/kg |
|---|---|---|---|---|---|---|---|
| Braunkohle | Lignite (brown coal) | 45–65 | 60–75 | 6.0–5.8 | 34-17 | 0.5-3 | <28,470 |
| Flammkohle | Flame coal | 40-45 | 75-82 | 6.0-5.8 | >9.8 | ~1 | <32,870 |
| Gasflammkohle | Gas flame coal | 35-40 | 82-85 | 5.8-5.6 | 9.8-7.3 | ~1 | <33,910 |
| Gaskohle | Gas coal | 28-35 | 85-87.5 | 5.6-5.0 | 7.3-4.5 | ~1 | <34,960 |
| Fettkohle | Fat coal | 19-28 | 87.5-89.5 | 5.0-4.5 | 4.5-3.2 | ~1 | <35,380 |
| Esskohle | Forge coal | 14-19 | 89.5-90.5 | 4.5-4.0 | 3.2-2.8 | ~1 | <35,380 |
| Magerkohle | Nonbaking coal | 10-14 | 90.5-91.5 | 4.0-3.75 | 2.8-3.5 | ~1 | 35,380 |
| Anthrazit | Anthracite | 7-12 | >91.5 | <3.75 | <2.5 | ~1 | <35,300 |
| Note, the percentages are percent by mass of the indicated elements | |||||||
The middle six grades in the table represent a progressive transition from the English-language sub-bituminous to bituminous coal, while the last class is an approximate equivalent to anthracite, but more inclusive (US anthracite has < 6% volatiles).
Cannel coal (sometimes called “candle coal”) is a variety of fine-grained, high-rank coal with significant hydrogen content. It consists primarily of “exinite” macerals, now termed “liptinite”.
Hilt’s law
Hilt’s law is a geological term that states that, in a small area, the deeper the coal, the higher its rank (grade). The law holds true if the thermal gradient is entirely vertical, but metamorphism may cause lateral changes of rank, irrespective of depth.
Content
| Substance | Content |
|---|---|
| Mercury (Hg) | 0.10±0.01 ppm[13] |
| Arsenic (As) | 1.4 – 71 ppm[14] |
| Selenium (Se) | 3 ppm[15] |
Early uses as fuel
The earliest recognized use is from the Shenyang area of China 4000 BC where Neolithic inhabitants had begun carving ornaments from black lignite.[16] Coal from the Fushun mine in northeastern China was used to smelt copper as early as 1000 BCE.[17] Marco Polo, the Italian who traveled to China in the 13th century, described coal as “black stones … which burn like logs”, and said coal was so plentiful, people could take three hot baths a week.[18] In Europe, the earliest reference to the use of coal as fuel is from the geological treatise On stones (Lap. 16) by the Greek scientist Theophrastus (circa 371–287 BC):[19][20]
Among the materials that are dug because they are useful, those known as anthrakes [coals] are made of earth, and, once set on fire, they burn like charcoal. They are found in Liguria … and in Elis as one approaches Olympia by the mountain road; and they are used by those who work in metals.
— Theophrastus, On Stones (16) translation
Outcrop coal was used in Britain during the Bronze Age (3000–2000 BC), where it has been detected as forming part of the composition of funeral pyres.[21][22] In Roman Britain, with the exception of two modern fields, “theRomans were exploiting coals in all the major coalfields in England and Wales by the end of the second century AD”.[23] Evidence of trade in coal (dated to about AD 200) has been found at the Roman settlement at Heronbridge, near Chester, and in the Fenlands of East Anglia, where coal from the Midlands was transported via the Car Dyke for use in drying grain.[24] Coal cinders have been found in the hearths of villas and Roman forts, particularly inNorthumberland, dated to around AD 400. In the west of England, contemporary writers described the wonder of a permanent brazier of coal on the altar of Minerva at Aquae Sulis (modern day Bath), although in fact easily accessible surface coal from what became the Somerset coalfield was in common use in quite lowly dwellings locally.[25] Evidence of coal’s use for iron-working in the city during the Roman period has been found.[26] InEschweiler, Rhineland, deposits of bituminous coal were used by the Romans for the smelting of iron ore.[23]
No evidence exists of the product being of great importance in Britain before the High Middle Ages, after about AD 1000.[27] Mineral coal came to be referred to as “seacoal” in the 13th century; the wharf where the material arrived in London was known as Seacoal Lane, so identified in a charter of King Henry III granted in 1253.[28] Initially, the name was given because much coal was found on the shore, having fallen from the exposed coal seams on cliffs above or washed out of underwater coal outcrops,[27] but by the time of Henry VIII, it was understood to derive from the way it was carried to London by sea.[29] In 1257–59, coal from Newcastle upon Tyne was shipped to London for the smiths and lime-burners buildingWestminster Abbey.[27] Seacoal Lane and Newcastle Lane, where coal was unloaded at wharves along the River Fleet, are still in existence.[30] (See Industrial processes below for modern uses of the term.)
These easily accessible sources had largely become exhausted (or could not meet the growing demand) by the 13th century, when underground extraction by shaft mining or adits was developed.[21] The alternative name was “pitcoal”, because it came from mines. It was, however, the development of the Industrial Revolution that led to the large-scale use of coal, as the steam engine took over from the water wheel. In 1700, five-sixths of the world’s coal was mined in Britain. Britain would have run out of suitable sites for watermills by the 1830s if coal had not been available as a source of energy.[31] In 1947, there were some 750,000 miners in Britain,[32] but by 2004, this had shrunk to some 5,000 miners working in around 20 collieries.[33]
Uses today

Castle Gate Power Plant nearHelper, Utah, USA
Coal as fuel
Coal is primarily used as a solid fuel to produce electricity and heat through combustion. World coal consumption was about 7.25 billion tonnes in 2010[34] (7.99 billion short tons) and is expected to increase 48% to 9.05 billion tonnes (9.98 billion short tons) by 2030.[35] China produced 3.47 billion tonnes (3.83 billion short tons) in 2011. India produced about 578 million tonnes (637.1 million short tons) in 2011. 68.7% of China’s electricity comes from coal. The USA consumed about 13% of the world total in 2010, i.e. 951 million tonnes (1.05 billion short tons), using 93% of it for generation of electricity.[36] 46% of total power generated in the USA was done using coal.[37] The United States Energy Information Administration estimates coal reserves at 948×109 short tons (860 Gt).[38] One estimate for resources is 18,000 Gt.[39]
When coal is used for electricity generation, it is usually pulverized and then combusted (burned) in a furnace with a boiler.[40] The furnace heat converts boiler water to steam, which is then used to spin turbines which turngenerators and create electricity.[41] The thermodynamic efficiency of this process has been improved over time; some older coal-fired power stations have thermal efficiencies in the vicinity of 25%[42] whereas the newestsupercritical and “ultra-supercritical” steam cycle turbines, operating at temperatures over 600 °C and pressures over 27 MPa (over 3900 psi), can practically achieve thermal efficiencies in excess of 45% (LHV basis) using anthracite fuel,[43][44] or around 43% (LHV basis) even when using lower-grade lignite fuel.[45] Further thermal efficiency improvements are also achievable by improved pre-drying (especially relevant with high-moisture fuel such as lignite or biomass) and cooling technologies.[46]
An alternative approach of using coal for electricity generation with improved efficiency is the integrated gasification combined cycle (IGCC) power plant. Instead of pulverizing the coal and burning it directly as fuel in the steam-generating boiler, the coal can be first gasified (see coal gasification) to create syngas, which is burned in a gas turbine to produce electricity (just like natural gas is burned in a turbine). Hot exhaust gases from the turbine are used to raise steam in a heat recovery steam generator which powers a supplemental steam turbine. Thermal efficiencies of current IGCC power plants range from 39-42%[47] (HHV basis) or ~42-45% (LHV basis) for bituminous coal and assuming utilization of mainstream gasification technologies (Shell, GE Gasifier, CB&I). IGCC power plants outperform conventional pulverized coal-fueled plants in terms of pollutant emissions, and allow for relatively easy carbon capture.
At least 40% of the world’s electricity comes from coal,[40][48] and in 2012, about one-third of the United States’ electricity came from coal, down from approximately 49% in 2008.[49][50] As of 2012 in the United States, use of coal to generate electricity was declining, as plentiful supplies of natural gas obtained by hydraulic fracturing of tight shale formations became available at low prices.[49]
In Denmark, a net electric efficiency of > 47% has been obtained at the coal-fired Nordjyllandsværket CHP Plant and an overall plant efficiency of up to 91% with cogeneration of electricity and district heating.[51] The multifuel-fired Avedøreværket CHP Plant just outside Copenhagen can achieve a net electric efficiency as high as 49%. The overall plant efficiency with cogeneration of electricity and district heating can reach as much as 94%.[52]
An alternative form of coal combustion is as coal-water slurry fuel (CWS), which was developed in the Soviet Union. CWS significantly reduces emissions, improving the heating value of coal.[citation needed] Other ways to use coal are combined heat and power cogeneration and an MHD topping cycle.
The total known deposits recoverable by current technologies, including highly polluting, low-energy content types of coal (i.e., lignite, bituminous), is sufficient for many years.[quantify] However, consumption is increasing and maximal production could be reachedwithin decades (see world coal reserves, below). On the other hand, much may have to be left in the ground to avoid climate change.[53][54]
Coking coal and use of coke

Coke oven at a smokeless fuel plant in Wales, United Kingdom
Coke is a solid carbonaceous residue derived from low-ash, low-sulfur bituminous coal (metallurgical coal), from which the volatile constituents are driven off by baking in an oven without oxygen at temperatures as high as 1,000 °C (1,832 °F), so the fixed carbon and residual ash are fused together. Metallurgical coke is used as a fuel and as a reducing agent in smelting iron ore in a blast furnace.[55] The result is pig iron, and is too rich in dissolved carbon, so it must be treated further to make steel. The coking coal should be low in sulfur and phosphorus, so they do not migrate to the metal. Based on the ash percentage, the coking coal can be divided into various grades. These grades are:
- Steel Grade I (Not exceeding 15%)
- Steel Grade II (Exceeding 15% but not exceeding 18%)
- Washery Grade I (Exceeding 18% but not exceeding 21%)
- Washery Grade II (Exceeding 21% but not exceeding 24%)
- Washery Grade III (Exceeding 24% but not exceeding 28%)
- Washery Grade IV (Exceeding 28% but not exceeding 35%)[56]
The coke must be strong enough to resist the weight of overburden in the blast furnace, which is why coking coal is so important in making steel using the conventional route. However, the alternative route is direct reduced iron, where any carbonaceous fuel can be used to make sponge or pelletised iron. Coke from coal is grey, hard, and porous and has a heating value of 24.8 million Btu/ton (29.6 MJ/kg). Some cokemaking processes produce valuable byproducts, including coal tar, ammonia, light oils, and coal gas.
Petroleum coke is the solid residue obtained in oil refining, which resembles coke, but contains too many impurities to be useful in metallurgical applications.
Gasification
Coal gasification can be used to produce syngas, a mixture of carbon monoxide (CO) and hydrogen (H2) gas. Often syngas is used to fire gas turbines to produce electricity, but the versatility of syngas also allows it to be converted into transportation fuels, such as gasoline and diesel, through the Fischer-Tropsch process; alternatively, syngas can be converted into methanol, which can be blended into fuel directly or converted to gasoline via the methanol to gasoline process.[57] Gasification combined with Fischer-Tropsch technology is currently used by the Sasol chemical company of South Africa to make motor vehicle fuels from coal and natural gas. Alternatively, the hydrogen obtained from gasification can be used for various purposes, such as powering a hydrogen economy, making ammonia, or upgrading fossil fuels.
During gasification, the coal is mixed with oxygen and steam while also being heated and pressurized. During the reaction, oxygen and water molecules oxidize the coal into carbon monoxide (CO), while also releasing hydrogen gas (H2). This process has been conducted in both underground coal mines and in the production of town gas.
- C (as Coal) + O2 + H2O → H2 + CO
If the refiner wants to produce gasoline, the syngas is collected at this state and routed into a Fischer-Tropsch reaction. If hydrogen is the desired end-product, however, the syngas is fed into the water gas shift reaction, where more hydrogen is liberated.
- CO + H2O → CO2 + H2
In the past, coal was converted to make coal gas (town gas), which was piped to customers to burn for illumination, heating, and cooking.
Liquefaction
Coal can also be converted into synthetic fuels equivalent to gasoline or diesel by several different direct processes (which do not intrinsically require gasification or indirect conversion).[58] In the direct liquefaction processes, the coal is either hydrogenated orcarbonized. Hydrogenation processes are the Bergius process,[59] the SRC-I and SRC-II (Solvent Refined Coal) processes, the NUS Corporation hydrogenation process[60][61] and several other single-stage and two-stage processes.[62] In the process of low-temperature carbonization, coal is coked at temperatures between 360 and 750 °C (680 and 1,380 °F). These temperatures optimize the production of coal tars richer in lighter hydrocarbons than normal coal tar. The coal tar is then further processed into fuels. An overview of coal liquefaction and its future potential is available.[63]
Coal liquefaction methods involve carbon dioxide (CO2) emissions in the conversion process. If coal liquefaction is done without employing either carbon capture and storage (CCS) technologies or biomass blending, the result is lifecycle greenhouse gas footprints that are generally greater than those released in the extraction and refinement of liquid fuel production from crude oil. If CCS technologies are employed, reductions of 5–12% can be achieved in Coal to Liquid (CTL) plants and up to a 75% reduction is achievable when co-gasifying coal with commercially demonstrated levels of biomass (30% biomass by weight) in coal/biomass-to-liquids plants.[64] For future synthetic fuel projects, carbon dioxide sequestration is proposed to avoid releasing CO2 into the atmosphere. Sequestration adds to the cost of production.
Refined coal
Refined coal is the product of a coal-upgrading technology that removes moisture and certain pollutants from lower-rank coals such as sub-bituminous and lignite (brown) coals. It is one form of several precombustion treatments and processes for coal that alter coal’s characteristics before it is burned. The goals of precombustion coal technologies are to increase efficiency and reduce emissions when the coal is burned. Depending on the situation, precombustion technology can be used in place of or as a supplement to postcombustion technologies to control emissions from coal-fueled boilers.
Industrial processes
Finely ground bituminous coal, known in this application as sea coal, is a constituent of foundry sand. While the molten metal is in the mould, the coal burns slowly, releasing reducing gases at pressure, and so preventing the metal from penetrating the pores of the sand. It is also contained in ‘mould wash’, a paste or liquid with the same function applied to the mould before casting.[65] Sea coal can be mixed with the clay lining (the “bod”) used for the bottom of a cupola furnace. When heated, the coal decomposes and the bod becomes slightly friable, easing the process of breaking open holes for tapping the molten metal.[66]
Production of chemicals
Coal is an important feedstock in production of a wide range of chemical fertilizers and other chemical products.[67] The main route to these products is coal gasification to produce syngas. Primary chemicals that are produced directly from the syngas include methanol, hydrogen and carbon monoxide, which are the chemical building blocks from which a whole spectrum of derivative chemicals are manufactured, including olefins, acetic acid,formaldehyde, ammonia, urea and others. The versatility of syngas as a precursor to primary chemicals and high-value derivative products provides the option of using relatively inexpensive coal to produce a wide range of valuable commodities.
Historically, production of chemicals from coal has been used since the 1950s and has become established in the market. According to the 2010 Worldwide Gasification Database,[68] a survey of current and planned gasifiers, from 2004 to 2007 chemical production increased its gasification product share from 37% to 45%. From 2008 to 2010, 22% of new gasifier additions were to be for chemical production.
Because the slate of chemical products that can be made via coal gasification can in general also use feedstocks derived from natural gas and petroleum, the chemical industry tends to use whatever feedstocks are most cost-effective. Therefore, interest in using coal tends to increase for higher oil and natural gas prices and during periods of high global economic growth that may strain oil and gas production. Also, production of chemicals from coal is of much higher interest in countries like South Africa, China, India and the United States where there are abundant coal resources. The abundance of coal combined with lack of natural gas resources in China is strong inducement for the coal to chemicals industry pursued there. In the United States, the best example of the industry is Eastman Chemical Company which has been successfully operating a coal-to-chemicals plant at its Kingsport, Tennessee, site since 1983. Similarly, Sasol has built and operated coal-to-chemicals facilities in South Africa.
Coal to chemical processes do require substantial quantities of water. As of 2013 much of the coal to chemical production was in the People’s Republic of China[69][70] where environmental regulation and water management[71] was weak.[72]
Coal industry
Coal as a traded commodity
In North America, Central Appalachian coal futures contracts are currently traded on the New York Mercantile Exchange (trading symbol QL). The trading unit is 1,550 short tons (1,410 t) per contract, and is quoted in U.S. dollars and cents per ton. Since coal is the principal fuel for generating electricity in the United States, coal futures contracts provide coal producers and the electric power industry an important tool for hedging and risk management.[73]
In addition to the NYMEX contract, the Intercontinental Exchange (ICE) has European (Rotterdam) and South African (Richards Bay) coal futures available for trading. The trading unit for these contracts is 5,000 tonnes (5,500 short tons), and are also quoted in U.S. dollars and cents per ton.[74]
The price of coal increased from around $30.00 per short ton in 2000 to around $150.00 per short ton as of September 2008. As of October 2008, the price per short ton had declined to $111.50. Prices further declined to $71.25 as of October 2010.[75] In early 2015, it was trading near $56/ton.[76]
Environmental and health effects

Aerial photograph of Kingston Fossil Plant coal fly ash slurry spill site taken the day after the event
Health effects
The use of coal as fuel causes adverse health impact and deaths.[77]
The deadly London smog was caused primarily by the heavy use of coal. In the United States coal-fired power plants were estimated in 2004 to cause nearly 24,000 premature deaths every year, including 2,800 from lung cancer.[78] Annual health costs in Europe from use of coal to generate electricity are €42.8 billion, or $55 billion.[79] Yet the disease and mortality burden of coal use today falls most heavily upon China.[80][81][82]
Breathing in coal dust causes coalworker’s pneumoconiosis which is known colloquially as “black lung”, so-called because the coal dust literally turns the lungs black from their usual pink color.[83] In the United States alone, it is estimated that 1500 former employees of the coal industry die every year from the effects of breathing in coal mine dust.[84]
Around 10% of coal is ash,[85] Coal ash is hazardous and toxic to human beings and other living things.[86] Coal ash contains the radioactive elements uranium and thorium. Coal ash and other solid combustion byproducts are stored locally and escape in various ways that expose those living near coal plants to radiation and environmental toxics.[87]
Huge amounts of coal ash and other waste is produced annually. In 2013, the US alone consumed on the order of 983 million short tonnes of coal per year.[88] Use of coal on this scale generates hundreds of millions of tons of ash and other waste products every year. These include fly ash, bottom ash, and flue-gas desulfurization sludge, that contain mercury, uranium, thorium, arsenic, and other heavy metals, along with non-metals such asselenium.[89]
The American Lung Association, the American Nurses’ Association, and the Physicians for Social Responsibility released a report in 2009 which details in depth the detrimental impact of the coal industry on human health, including workers in the mines and individuals living in communities near plants burning coal as a power source. This report provides medical information regarding damage to the lungs, heart, and nervous system of Americans caused by the burning of coal as fuel. It details how the air pollution caused by the plume of coal smokestack emissions is a cause of asthma, strokes, reduced intelligence, artery blockages, heart attacks, congestive heart failure, cardiac arrhythmias, mercury poisoning, arterial occlusion, and lung cancer.[2]
More recently, the Chicago School of Public Health released a largely similar report, echoing many of the same findings.[90]
Though coal burning has increasingly been supplanted by less-toxic natural gas use in recent years, a 2010 study by the Clean Air Task Force still estimated that “air pollution from coal-fired power plants accounts for more than 13,000 premature deaths, 20,000 heart attacks, and 1.6 million lost workdays in the U.S. each year.” The total monetary cost of these health impacts is over $100 billion annually.[91]
The WHO classifies coal as a “dirty fuel” and encourages the movement away from such fuels towards cleaner alternatives.
Environmental effects
Coal mining and coal fueling of power station and industrial processes can cause major environmental damage.[92]
Water systems are affected by coal mining coal.[93] For example, mining affects with groundwater and water table levels and acidity. Spills of fly ash, such as the Kingston Fossil Plant coal fly ash slurry spill, can also contaminate land and waterways, and destroy homes. Power stations that burn coal also consume large quantities of water. This can affect the flows of rivers, and has consequential impacts on other land uses.
One of the earliest known impacts of coal on the water cycle was acid rain. Approximately 75 Tg/S per year of sulfur dioxide (SO2) is released from burning coal. After release, the sulfur dioxide is oxidized to gaseous H2SO2 which scatters solar radiation, hence its increase in the atmosphere exerts a cooling effect on climate. This beneficially masks some of the warming caused by increased greenhouse gases. However, the sulphur is precipitated out of the atmosphere as acid rain in a matter of weeks,[94] whereas carbon dioxide remains in the atmosphere for hundreds of years. Release of SO2 also contributes to the widespread acidification of ecosystems.[95]
Disused coal mines can also cause issues. Subsidence can occur above tunnels, causing damage to infrastructure or cropland. Coal mining can also cause long lasting fires, and it has been estimated that around 1000 coal seam fires are burning at any given time[citation needed]. For example, there is a coal seam fire in Germany that has been burning since 1668, and is still burning in the 21st century.
Some environmental impacts are modest, such as dust nuisance. However, perhaps the largest and most long term effect of coal use is the release of carbon dioxide, a greenhouse gas that causes climate change and global warming, according to the IPCC and theEPA. Coal is the largest contributor to the human-made increase of CO2 in the atmosphere.[96]
The production of coke from coal produces ammonia, coal tar, and gaseous compounds as by-products which if discharged to land, air or waterways can act as environmental pollutants.[97] The Whyalla steelworks is one example of a coke producing facility where liquid ammonia is discharged to the marine environment.
In 1999, world gross carbon dioxide emissions from coal usage were 8,666 million tonnes of carbon dioxide.[98] In 2011, world gross emissions from coal usage were 14,416 million tonnes.[99] For every megawatt-hour generated, coal-fired electric power generation emits around 2,000 pounds of carbon dioxide, which is almost double the approximately 1100 pounds of carbon dioxide released by a natural gas-fired electric plant.[100] Because of this higher carbon efficiency of natural gas generation, as the market in the United States has changed to reduce coal and increase natural gas generation, carbon dioxide emissions may have fallen.[101] Those measured in the first quarter of 2012 were the lowest of any recorded for the first quarter of any year since 1992.[102] In 2013, the head of the UN climate agency advised that most of the world’s coal reserves should be left in the ground to avoid catastrophic global warming.[103]
Clean coal technology
“Clean” coal technology is a collection of technologies being developed to mitigate the environmental impact of coal energy generation.[104] Those technologies are being developed to remove or reduce pollutant emissions to the atmosphere. Some of the techniques that would be used to accomplish this include chemically washing minerals and impurities from the coal, gasification (see also IGCC), improved technology for treating flue gases to remove pollutants to increasingly stringent levels and at higher efficiency, carbon capture and storage technologies to capture the carbon dioxide from the flue gas and dewatering lower rank coals (brown coals) to improve the calorific value, and thus the efficiency of the conversion into electricity. Figures from the United States Environmental Protection Agency show that these technologies have made today’s coal-based generating fleet 77 percent cleaner on the basis of regulated emissions per unit of energy produced.[105]
Clean coal technology usually addresses atmospheric problems resulting from burning coal. Historically, the primary focus was on SO2 and NOx, the most important gases in causation of acid rain, and particulates which cause visible air pollution and deleterious effects on human health. More recent focus has been on carbon dioxide (due to its impact on global warming)[106] and concern over toxic species such as mercury.[107] Concerns exist regarding the economic viability of these technologies and the timeframe of delivery,[108] potentially high hidden economic costs in terms of social and environmental damage,[109] and the costs and viability of disposing of removed carbon and other toxic matter.[110][111]
Several different technological methods are available for the purpose of carbon capture as demanded by the clean coal concept:
- Pre-combustion capture – This involves gasification of a feedstock (such as coal) to form synthesis gas, which may be shifted to produce a H2 and CO2-rich gas mixture, from which the CO2 can be efficiently captured and separated, transported, and ultimately sequestered,[112] This technology is usually associated with Integrated Gasification Combined Cycle process configurations.[113]
- Post-combustion capture – This refers to capture of CO2 from exhaust gases of combustion processes, typically using sorbents, solvents, or membrane separations to remove CO2 from the bulk gases.[114]
- Oxy-fuel combustion – Fossil fuels such as coal are burned in a mixture of recirculated flue gas and oxygen, rather than in air, which largely eliminates nitrogen from the flue gas enabling efficient, low-cost CO2 capture.[115]
The Kemper County IGCC Project, a 582 MW coal gasification-based power plant, will use pre-combustion capture of CO2 to capture 65% of the CO2 the plant produces, which will be utilized/geologically sequestered inenhanced oil recovery operations.[116]
The Saskatchewan Government’s Boundary Dam Integrated Carbon Capture and Sequestration Demonstration Project will use post-combustion, amine-based scrubber technology to capture 90% of the CO2 emitted by Unit 3 of the power plant; this CO2 will be pipelined to and utilized for enhanced oil recovery in the Weyburn oil fields.[117]
An early example of a coal-based plant using (oxy-fuel) carbon-capture technology is Swedish company Vattenfall‘s Schwarze Pumpe power station located in Spremberg, Germany, built by German firm Siemens, which went on-line in September 2008.[118][119] The facility captures CO2 and acid rain producing pollutants, separates them, and compresses the CO2 into a liquid. Plans are to inject the CO2 into depleted natural gas fields or other geological formations. Vattenfall opines that this technology is considered not to be a final solution for CO2 reduction in the atmosphere, but provides an achievable solution in the near term while more desirable alternative solutions to power generation can be made economically practical.[119]
Bioremediation
The white rot fungus Trametes versicolor can grow on and metabolize naturally occurring coal.[120] The bacteria Diplococcus has been found to degrade coal, raising its temperature.[121]
Economic aspects
Coal (by liquefaction technology) is one of the backstop resources that could limit escalation of oil prices and mitigate the effects of transportation energy shortage that will occur under peak oil. This is contingent on liquefaction production capacity becoming large enough to satiate the very large and growing demand for petroleum. Estimates of the cost of producing liquid fuels from coal suggest that domestic U.S. production of fuel from coal becomes cost-competitive with oil priced at around $35 per barrel,[122] with the $35 being the break-even cost. With oil prices as low as around $40 per barrel in the U.S. as of December 2008, liquid coal lost some of its economic allure in the U.S., but will probably be re-vitalized, similar to oil sand projects, with an oil price around $70 per barrel.
In China, due to an increasing need for liquid energy in the transportation sector, coal liquefaction projects were given high priority even during periods of oil prices below $40 per barrel.[123] This is probably because China prefers not to be dependent on foreign oil, instead utilizing its enormous domestic coal reserves. As oil prices were increasing during the first half of 2009, the coal liquefaction projects in China were again boosted, and these projects are profitable with an oil barrel price of $40.[124]
China is the largest producer of coal in the world. It is the world’s largest energy consumer, and relies on coal to supply 69% of its energy needs.[125] An estimated 5 million people worked in China’s coal-mining industry in 2007.[126]
Coal pollution costs the EU €43 billion each year.[127] Measures to cut air pollution may have beneficial long-term economic impacts for individuals.[128]
Energy density and carbon impact
The energy density of coal, i.e. its heating value, is roughly 24 megajoules per kilogram[129] (approximately 6.7 kilowatt-hours per kg). For a coal power plant with a 40% efficiency, it takes an estimated 325 kg (717 lb) of coal to power a 100 W lightbulb for one year.[130]
As of 2006, the average efficiency of electricity-generating power stations was 31%; in 2002, coal represented about 23% of total global energy supply, an equivalent of 3.4 billion tonnes of coal, of which 2.8 billion tonnes were used for electricity generation.[131]
The US Energy Information Agency’s 1999 report on CO2 emissions for energy generation quotes an emission factor of 0.963 kg CO2/kWh for coal power, compared to 0.881 kg CO2/kWh (oil), or 0.569 kg CO2/kWh (natural gas).[132]
Underground fires
Thousands of coal fires are burning around the world.[133] Those burning underground can be difficult to locate and many cannot be extinguished. Fires can cause the ground above to subside, their combustion gases are dangerous to life, and breaking out to the surface can initiate surface wildfires. Coal seams can be set on fire by spontaneous combustion or contact with a mine fire or surface fire. Lightning strikes are an important source of ignition. The coal continues to burn slowly back into the seam until oxygen (air) can no longer reach the flame front. A grass fire in a coal area can set dozens of coal seams on fire.[134][135] Coal fires in China burn an estimated 120 million tons of coal a year, emitting 360 million metric tons of CO2, amounting to 2–3% of the annual worldwide production of CO2 from fossil fuels.[136][137] In Centralia, Pennsylvania (a borough located in the Coal Region of the United States), an exposed vein of anthracite ignited in 1962 due to a trash fire in the borough landfill, located in an abandoned anthracite strip minepit. Attempts to extinguish the fire were unsuccessful, and it continues to burn underground to this day. The Australian Burning Mountain was originally believed to be a volcano, but the smoke and ash come from a coal fire that has been burning for some 6,000 years.[138]
At Kuh i Malik in Yagnob Valley, Tajikistan, coal deposits have been burning for thousands of years, creating vast underground labyrinths full of unique minerals, some of them very beautiful. Local people once used this method[clarification needed] to mine ammoniac. This place has been well-known since the time of Herodotus, but European geographers misinterpreted the Ancient Greek descriptions as the evidence of active volcanism in Turkestan (up to the 19th century, when the Russian army invaded the area).[citation needed]
The reddish siltstone rock that caps many ridges and buttes in the Powder River Basin in Wyoming and in western North Dakota is called porcelanite, which resembles the coal burning waste “clinker” or volcanic “scoria“.[139] Clinker is rock that has been fused by the natural burning of coal. In the Powder River Basin approximately 27 to 54 billion tons of coal burned within the past three million years.[140] Wild coal fires in the area were reported by the Lewis and Clark Expedition as well as explorers and settlers in the area.[141]
Production trends

A coal mine in Wyoming, United States. The United States has the world’s largest coal reserves.
In 2006, China was the top producer of coal with 38% share followed by the United States and India, according to the British Geological Survey. As of 2012 coal production in the United States was falling at the rate of 7% annually[142] with many power plants using coal shut down or converted to natural gas; however, some of the reduced domestic demand was taken up by increased exports[143] with five coal export terminals being proposed in the Pacific Northwest to export coal from the Powder River Basin to China and other Asian markets;[144] however, as of 2013, environmental opposition was increasing.[145] High-sulfur coal mined in Illinois which was unsaleable in the United States found a ready market in Asia as exports reached 13 million tons in 2012.[146]
World coal reserves
The 948 billion short tons of recoverable coal reserves estimated by the Energy Information Administration are equal to about 4,196 BBOE (billion barrels of oil equivalent).[38] The amount of coal burned during 2007 was estimated at 7.075 billion short tons, or 133.179 quadrillion BTU’s.[147] This is an average of 18.8 million BTU per short ton. In terms of heat content, this is about 57,000,000 barrels (9,100,000 m3) of oil equivalent per day. By comparison in 2007, natural gas provided 51,000,000 barrels (8,100,000 m3) of oil equivalent per day, while oil provided 85,800,000 barrels (13,640,000 m3) per day.
British Petroleum, in its 2007 report, estimated at 2006 end that there were 147 years reserves-to-production ratio based on proven coal reserves worldwide. This figure only includes reserves classified as “proven”; exploration drilling programs by mining companies, particularly in under-explored areas, are continually providing new reserves. In many cases, companies are aware of coal deposits that have not been sufficiently drilled to qualify as “proven”. However, some nations haven’t updated their information and assume reserves remain at the same levels even with withdrawals.
Of the three fossil fuels, coal has the most widely distributed reserves; coal is mined in over 100 countries, and on all continents except Antarctica. The largest reserves are found in the United States, Russia, China, Australia and India. Note the table below.
| Country | Anthracite & Bituminous | SubBituminous | Lignite | Total | Percentage of World Total | Year |
|---|---|---|---|---|---|---|
| 108,501 | 98,618 | 30,176 | 237,295 | 22.6 | 2011 | |
| 49,088 | 97,472 | 10,450 | 157,010 | 14.4 | 2011 | |
| 62,200 | 33,700 | 18,600 | 114,500 | 12.6 | 2011 | |
| 37,100 | 2,100 | 37,200 | 76,400 | 8.9 | 2011 | |
| 56,100 | 0 | 4,500 | 60,600 | 7.0 | 2011 | |
| 99 | 0 | 40,600 | 40,699 | 4.7 | ||
| 15,351 | 16,577 | 1,945 | 33,873 | 3.9 | ||
| 21,500 | 0 | 12,100 | 33,600 | 3.9 | ||
| 30,156 | 0 | 0 | 30,156 | 3.5 | ||
| 9 | 361 | 13,400 | 13,770 | 1.6 | ||
| 6,366 | 380 | 0 | 6,746 | 0.8 | ||
| 3,474 | 872 | 2,236 | 6,528 | 0.8 | ||
| 4,338 | 0 | 1,371 | 5,709 | 0.7 | ||
| 1,520 | 2,904 | 1,105 | 5,529 | 0.6 | ||
| 0 | 4,559 | 0 | 4,559 | 0.5 | ||
| 0 | 0 | 3,020 | 3,020 | 0.4 | ||
| 484 | 0 | 2,369 | 2,853 | 0.3 | ||
| 1,170 | 0 | 1,350 | 2,520 | 0.3 | ||
| 2 | 190 | 2,174 | 2,366 | 0.3 | ||
| 0 | 166 | 1,904 | 2,070 | 0.3 | ||
| 529 | 0 | 1,814 | 2,343 | 0.3 | ||
| 47 | 0 | 1,853 | 1,900 | 0.2 | ||
| 13 | 439 | 1,208 | 1,660 | 0.2 | ||
| 0 | 0 | 1,239 | 1,239 | 0.1 | ||
| 860 | 300 | 51 | 1,211 | 0.1 | ||
| 1,203 | 0 | 0 | 1,203 | 0.1 | ||
| 192 | 0 | 908 | 1,100 | 0.1 | ||
| 0 | 0 | 812 | 812 | 0.1 | ||
| 0 | 0 | 794 | 794 | 0.1 | ||
| 300 | 300 | 0 | 600 | 0.1 | ||
| 33 | 205 | 333-7,000 | 571–15,000[149] | 0.1 | ||
| 200 | 300 | 30 | 530 | 0.1 | ||
| 4 | 0 | 499 | 503 | 0.1 | ||
| 502 | 0 | 0 | 502 | 0.1 | ||
| 0 | 0 | 550 | 550 | 0.1 | 2011 | |
| All others | 3,421 | 1,346 | 846 | 5,613 | 0.7 | |
| World Total | 403,197 | 287,333 | 201,000 | 891,530 | 100 | 2011 |
Major coal producers
The reserve life is an estimate based only on current production levels and proved reserves level for the countries shown, and makes no assumptions of future production or even current production trends. Countries with annual production higher than 100 million tonnes are shown. For comparison, data for the European Union is also shown. Shares are based on data expressed in tonnes oil equivalent.
| Country | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | Share | Reserve Life (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1834.9 | 2122.6 | 2349.5 | 2528.6 | 2691.6 | 2802.0 | 2973.0 | 3235.0 | 3520.0 | 49.5% | 35 | |
| 972.3 | 1008.9 | 1026.5 | 1054.8 | 1040.2 | 1063.0 | 975.2 | 983.7 | 992.8 | 14.1% | 239 | |
| 375.4 | 407.7 | 428.4 | 449.2 | 478.4 | 515.9 | 556.0 | 573.8 | 588.5 | 5.6% | 103 | |
| 637.2 | 627.6 | 607.4 | 595.1 | 592.3 | 563.6 | 538.4 | 535.7 | 576.1 | 4.2% | 97 | |
| 350.4 | 364.3 | 375.4 | 382.2 | 392.7 | 399.2 | 413.2 | 424.0 | 415.5 | 5.8% | 184 | |
| 276.7 | 281.7 | 298.3 | 309.9 | 313.5 | 328.6 | 301.3 | 321.6 | 333.5 | 4.0% | 471 | |
| 114.3 | 132.4 | 152.7 | 193.8 | 216.9 | 240.2 | 256.2 | 275.2 | 324.9 | 5.1% | 17 | |
| 237.9 | 243.4 | 244.4 | 244.8 | 247.7 | 252.6 | 250.6 | 254.3 | 255.1 | 3.6% | 118 | |
| 204.9 | 207.8 | 202.8 | 197.1 | 201.9 | 192.4 | 183.7 | 182.3 | 188.6 | 1.1% | 216 | |
| 163.8 | 162.4 | 159.5 | 156.1 | 145.9 | 144.0 | 135.2 | 133.2 | 139.2 | 1.4% | 41 | |
| 84.9 | 86.9 | 86.6 | 96.2 | 97.8 | 111.1 | 100.9 | 110.9 | 115.9 | 1.5% | 290 | |
| World Total | 5,301.3 | 5,716.0 | 6,035.3 | 6,342.0 | 6,573.3 | 6,795.0 | 6,880.8 | 7,254.6 | 7,695.4 | 100% | 112 |
Major coal consumers
Countries with annual consumption higher than 20 million tonnes are shown.
| Country | 2008 | 2009 | 2010 | 2011 | Share |
|---|---|---|---|---|---|
| 2,966 | 3,188 | 3,695 | 4,053 | 50.7% | |
| 1,121 | 997 | 1,048 | 1,003 | 12.5% | |
| 641 | 705 | 722 | 788 | 9.9% | |
| 250 | 204 | 256 | 262 | 3.3% | |
| 268 | 248 | 256 | 256 | 3.3% | |
| 215 | 204 | 206 | 210 | 2.6% | |
| 204 | 181 | 206 | 202 | 2.5% | |
| 149 | 151 | 149 | 162 | 2.0% | |
| World Total | 7,327 | 7,318 | 7,994 | N/A | 100% |
Major coal exporters
Countries with annual gross export higher than 10 million tonnes are shown. In terms of net export the largest exporters are still Australia (328.1 millions tonnes), Indonesia (316.2) and Russia (100.2).
| Country | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | Share |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 107.8 | 131.4 | 142.0 | 192.2 | 221.9 | 228.2 | 261.4 | 316.2 | 331.4 | 421.8 | 29.8% | |
| 238.1 | 247.6 | 255.0 | 255.0 | 268.5 | 278.0 | 288.5 | 328.1 | 313.6 | 332.4 | 23.5% | |
| 41.0 | 55.7 | 98.6 | 103.4 | 112.2 | 115.4 | 130.9 | 122.1 | 140.1 | 150.7 | 10.7% | |
| 43.0 | 48.0 | 51.7 | 51.2 | 60.6 | 83.5 | 60.4 | 83.2 | 108.2 | 126.7 | 8.7% | |
| 50.4 | 56.4 | 59.2 | 68.3 | 74.5 | 74.7 | 75.7 | 76.4 | 89.0 | 92.2 | 6.5% | |
| 78.7 | 74.9 | 78.8 | 75.8 | 72.6 | 68.2 | 73.8 | 76.7 | 75.8 | 82.0 | 5.8% | |
| 27.7 | 28.8 | 31.2 | 31.2 | 33.4 | 36.5 | 31.9 | 36.9 | 37.6 | 38.8 | 2.7% | |
| 30.3 | 27.4 | 28.3 | 30.5 | 32.8 | 47.6 | 33.0 | 36.3 | 33.5 | 35.2 | 2.5% | |
| 0.5 | 1.7 | 2.3 | 2.5 | 3.4 | 4.4 | 7.7 | 18.3 | 26.1 | 24.3 | 1.7% | |
| 6.9 | 11.7 | 19.8 | 23.5 | 35.1 | 21.3 | 28.2 | 24.7 | 19.7 | 21.1 | 1.5% | |
| 103.4 | 95.5 | 93.1 | 85.6 | 75.4 | 68.8 | 25.2 | 22.7 | 27.5 | 15.2 | 1.1% | |
| 28.0 | 27.5 | 26.5 | 25.4 | 20.1 | 16.1 | 14.6 | 18.1 | 15.0 | 14.9 | 1.0% | |
| Total World | 713.9 | 764.0 | 936.0 | 1,000.6 | 1,073.4 | 1,087.3 | 1,090.8 | 1,212.8 | 1,286.7 | 1,413.9 | 100% |
Major coal importers
Countries with annual gross import higher than 20 million tonnes are shown. In terms of net import the largest importers are still Japan (206.0 millions tonnes), China (172.4) and South Korea (125.8).[153]
| Country | 2006 | 2007 | 2008 | 2009 | 2010 | Share |
|---|---|---|---|---|---|---|
| 199.7 | 209.0 | 206.0 | 182.1 | 206.7 | 17.5% | |
| 42.0 | 56.2 | 44.5 | 151.9 | 195.1 | 16.6% | |
| 84.1 | 94.1 | 107.1 | 109.9 | 125.8 | 10.7% | |
| 52.7 | 29.6 | 70.9 | 76.7 | 101.6 | 8.6% | |
| 69.1 | 72.5 | 70.9 | 64.6 | 71.1 | 6.0% | |
| 50.6 | 56.2 | 55.7 | 45.9 | 55.1 | 4.7% | |
| 22.9 | 25.8 | 21.7 | 22.7 | 30.0 | 2.5% | |
| 56.8 | 48.9 | 49.2 | 42.2 | 29.3 | 2.5% | |
| 27.9 | 28.0 | 27.9 | 20.9 | 23.7 | 1.9% | |
| 25.7 | 29.3 | 23.5 | 22.1 | 22.8 | 1.9% | |
| 28.8 | 26.3 | 34.6 | 26.8 | 21.8 | 1.9% | |
| 24.1 | 22.1 | 24.9 | 18.3 | 20.8 | 1.8% | |
| 40.3 | 38.8 | 37.8 | 23.1 | 20.6 | 1.8% | |
| Total | 991.8 | 1,056.5 | 1,063.2 | 1,039.8 | 1,178.1 | 100% |
|
||
Cultural usage
Coal is the official state mineral of Kentucky.[154] and the official state rock of Utah;[155] both U.S. states have a historic link to coal mining.
Some cultures hold that children who misbehave will receive only a lump of coal from Santa Claus for Christmas in their christmas stockings instead of presents.
It is also customary and considered lucky in Scotland and the North of England to give coal as a gift on New Year’s Day. This occurs as part of First-Footing and represents warmth for the year to come.
See also
- Abiogenic petroleum origin
- Asphaltene
- Biochar
- Biomass-coal
- Carbochemistry
- Clean coal
- Coal assay
- Coal Blending
- Coal homogenization
- Coal Measure (stratigraphic unit)
- Coal phase out
- Coal-tar
- Coalbed methane
- Fluidized bed combustion
- Fossil fuels
- Gytta
- Major coal producing regions
- Mountaintop removal mining
- Oil (petroleum)
- The Coal Question
- Tonstein
- World Coal Association
References
- Jump up^ Blander, M. “Calculations of the Influence of Additives on Coal Combustion Deposits” (PDF). Argonne National Laboratory. p. 315. Retrieved 17 December 2011.
- ^ Jump up to:a b http://www.psr.org/news-events/press-releases/coal-pollution-damages-human-health.html.
- ^ Jump up to:a b c “BP Statistical review of world energy 2012”. British Petroleum. Archived from the original (XLS) on 19 June 2012. Retrieved 18 August 2011.
- ^ Jump up to:a b EIA International Energy Annual – Total Coal Exports (Thousand Short Tons). Tonto.eia.doe.gov. Retrieved on 24 August 2012.
- ^ Jump up to:a b International Energy Annual – Total Coal Imports (Thousand Short Tons). Tonto.eia.doe.gov. Retrieved on 24 August 2012.
- ^ Jump up to:a b c Harper, Douglas. “coal”. Online Etymology Dictionary.
- Jump up^ Gerard Clauson: An Etymological Dictionary of Pre-Thirteenth Century Turkish, Oxford at the Clarendon Press, 1972, pp. 19, 715
- Jump up^ Taylor, Thomas N; Taylor, Edith L; Krings, Michael (2009).Paleobotany: The biology and evolution of fossil plants.ISBN 978-0-12-373972-8.
- Jump up^ Tyler, S. A.; Barghoorn, E. S.; Barrett, L. P. (1957). “Anthracitic Coal from Precambrian Upper Huronian Black Shale of the Iron River District, Northern Michigan”. Geological Society of America Bulletin 68 (10): 1293. doi:10.1130/0016-7606(1957)68[1293:ACFPUH]2.0.CO;2. ISSN 0016-7606.
- Jump up^ Mancuso, J. J.; Seavoy, R. E. (1981). “Precambrian coal or anthraxolite; a source for graphite in high-grade schists and gneisses”. Economic Geology 76 (4): 951–954.doi:10.2113/gsecongeo.76.4.951.
- Jump up^ Funk and Wagnalls, quoted in “sea-coal”. Oxford English Dictionary (2 ed.). Oxford University Press. 1989.
- Jump up^ Eberhard Lindner; Chemie für Ingenieure; Lindner Verlag Karlsruhe, S. 258
- Jump up^ “Mercury in coal: a review ; Part 1. Geochemistry ; Ya. E. Yudovich*, M.P. Ketris”. labtechgroup.com. 2010-04-21. Archived from the original (PDF) on 2013-03-23. Retrieved2013-02-22.
- Jump up^ “Arsenic in Coal” (PDF). pubs.usgs.gov. 2006-03-28. Retrieved 2013-02-22.
- Jump up^ “Selenium in Our Environment – Trace Elements in the Environment – Advances in Chemistry (ACS Publications)”. pubs.acs.org. 1973-06-01. doi:10.1021/ba-1973-0123.ch006.
- Jump up^ [“Science and Civilisation in China” by Peter J Golas and Joseph Needham. p. 186-191. Cambridge University Press 1999.ISBN 0-521-58000-5, 978-0-521-58000-7. Google Books]
- Jump up^ coal. Encyclopedia Britannica.
- Jump up^ Marco Polo In China. Facts and Details. Retrieved on 2013-05-11. Archived 21 September 2013 at the Wayback Machine.
- Jump up^ Carol, Mattusch (2008). Oleson, John Peter, ed. Metalworking and Tools. The Oxford Handbook of Engineering and Technology in the Classical World (Oxford University Press). pp. 418–38 (432). ISBN 978-0-19-518731-1
- Jump up^ Irby-Massie, Georgia L.; Keyser, Paul T. (2002). Greek Science of the Hellenistic Era: A Sourcebook. Routledge. 9.1 “Theophrastos”, p.228. ISBN 0-415-23847-1
- ^ Jump up to:a b Britannica 2004: Coal mining: ancient use of outcropping coal
- Jump up^ Needham, Joseph; Golas, Peter J (1999). Science and Civilisation in China. Cambridge University Press. pp. 186–91.ISBN 978-0-521-58000-7.
- ^ Jump up to:a b Smith, A. H. V. (1997). “Provenance of Coals from Roman Sites in England and Wales”. Britannia 28: 297–324 (322–4).doi:10.2307/526770. JSTOR 526770.
- Jump up^ Salway, Peter (2001). A History of Roman Britain. Oxford University Press. ISBN 0-19-280138-4.
- Jump up^ Forbes, RJ (1966): Studies in Ancient Technology. Brill Academic Publishers, Boston.
- Jump up^ Cunliffe, Barry W. (1984). Roman Bath Discovered. London: Routledge. pp. 14–5, 194. ISBN 0-7102-0196-6.
- ^ Jump up to:a b c Cantril, T. C. (1914). Coal Mining. Cambridge, England: Cambridge University Press. pp. 3–10. OCLC 156716838.
- Jump up^ “coal, 5a”. Oxford English Dictionary. Oxford University Press. 1 December 2010.
- Jump up^ John Caius, quoted in Cantril (1914).
- Jump up^ Trench, Richard; Hillman, Ellis (1993). London under London: a subterranean guide (Second ed.). London: John Murray. p. 33.ISBN 0-7195-5288-5.
- Jump up^ Wrigley, EA (1990). Continuity, Chance and Change: The Character of the Industrial Revolution in England. Cambridge University Press. ISBN 0-521-39657-3.
- Jump up^ “The fall of King Coal”. BBC News. 6 December 1999.
- Jump up^ “The miners’ strike revisited”. Inside Out (BBC). 2 February 2004.
- Jump up^ World coal consumption 2000–2011 EIA statistics
- Jump up^ EIA, World Energy Projections Plus (2009)
- Jump up^ U.S. Coal Supply and Demand: 2010 Year in Review. Eia.gov (2011-06-01). Retrieved on 24 August 2012.
- Jump up^ Share of electric power sector net generation by energy source. Eia.gov (2011-06-01). Retrieved on 24 August 2012.
- ^ Jump up to:a b “EIA International Energy Statistics : Coal : Recoverable Reserves”. Retrieved 31 May 2012.
- Jump up^ Fred Pearce (Feb 15, 2014). “Fire in the hole: After fracking comes coal”. New Scientist: 36–41.
- ^ Jump up to:a b Total World Electricity Generation by Fuel (2006). Source: IEA 2008.
- Jump up^ “Fossil Power Generation”. Siemens AG. Retrieved 23 April2009.[dead link]
- Jump up^ J. Nunn, A. Cottrell, A. Urfer, L. Wibberley and P. Scaife, “A Lifecycle Assessment of the Victorian Energy Grid”, Cooperative Research Centre for Coal in Sustainable Development, February 2003, page 7.
- Jump up^ Jens Rosenkranz; Andreas Wichtmann. “Balancing economics and environmental friendliness – the challenge for supercritical coal-fired power plants with highest steam parameters in the future” (PDF). Retrieved 23 October 2006.
- Jump up^ “Lünen – State-of-theArt Ultra Supercritical Steam Power Plant Under Construction” (PDF). Siemens AG. Retrieved 21 July2014.
- Jump up^ “Neurath F and G set new benchmarks” (PDF). Alstom. Retrieved 21 July 2014.
- Jump up^ “The Niederraussem Coal Innovation Centre” (PDF). RWE. Retrieved 21 July 2014.
- Jump up^ “IGCC Efficiency/Performance”. National Energy Technology Laboratory. Retrieved 16 July 2014.
- Jump up^ coal reserves, coal exploration – World Coal Association. Worldcoal.org. Retrieved on 24 August 2012.
- ^ Jump up to:a b Lipton, Eric (29 May 2012). “Even in Coal Country, the Fight for an Industry”. The New York Times. Retrieved 30 May 2012.
- Jump up^ “Figure ES 1. U.S. Electric Power Industry Net Generation”.Electric Power Annual with data for 2008. U.S. Energy Information Administration. 21 January 2010. Retrieved7 November 2010.
- Jump up^ Nordjyllandsværket. (PDF) . Retrieved on 2013-05-11.Archived 20 November 2012 at the Wayback Machine.
- Jump up^ Avedøreværket. Ipaper.ipapercms.dk. Retrieved on 2013-05-11.
- Jump up^ Michael Slezak. “Mining insider: ‘Leave the coal in the ground’ – New Scientist”. New Scientist. Retrieved 7 January 2016.
- Jump up^ “IPCC digested: Just leave the fossil fuels underground”. New Scientist.
- Jump up^ Blast furnace steelmaking cost model. Steelonthenet.com. Retrieved on 24 August 2012.
- Jump up^ “Coal Grades”,”Ministry of Coal”
- Jump up^ “Conversion of Methanol to Gasoline”. National Energy Technology Laboratory. Retrieved 2014-07-16.
- Jump up^ “Direct Liquefaction Processes”. National Energy Technology Laboratory. Retrieved 2014-07-16.
- Jump up^ Haul, Robert (April 1985). “Friedrich Bergius (1884–1949)”.Chemie in unserer Zeit (VCH-Verlagsgesellschaft mbH) 19: 62.doi:10.1002/ciuz.19850190205.
- Jump up^ Speight, James G. (2008). Synthetic Fuels Handbook: Properties, Process, and Performance. McGraw-Hill Professional. pp. 9–10. ISBN 978-0-07-149023-8.
- Jump up^ Lowe, Phillip A.; Schroeder, Wilburn C.; Liccardi, Anthony L. (1976). “Technical Economies, Synfuels and Coal Energy Symposium, Solid-Phase Catalytic Coal Liquefaction Process”.American Society of Mechanical Engineers: 35.
- Jump up^ “Direct Liquefaction Processes, Single-stage Processes and Two-stage Processes”. National Energy Technology Laboratory. Retrieved 2014-07-16.
- Jump up^ Höök, M., Aleklett, K. (2010). “A review on coal-to-liquid fuels and its coal consumption”. International Journal of Energy Research 34 (10): 848. doi:10.1002/er.1596. Archived from the original (PDF) on 2010-02-21.
- Jump up^ Tarka, Thomas J.; Wimer, John G.; Balash, Peter C.; Skone, Timothy J.; Kern, Kenneth C.; Vargas, Maria C.; Morreale, Bryan D.; White III, Charles W.; Gray, David (2009). “Affordable Low Carbon Diesel from Domestic Coal and Biomass”. United States Department of Energy, National Energy Technology Laboratory: 21.
- Jump up^ Rao, P. N. (2007). “Moulding materials”. Manufacturing technology: foundry, forming and welding (2 ed.). New Delhi: Tata McGraw-Hill. p. 107. ISBN 978-0-07-463180-5.
- Jump up^ Kirk, Edward (1899). “Cupola management”. Cupola Furnace – A Practical Treatise on the Construction and Management of Foundry Cupolas. Philadelphia, PA: Baird. p. 95.OCLC 2884198.
- Jump up^ “Chemicals”. National Energy Technology Laboratory. Retrieved 12 July 2014.
- Jump up^ “Gasification Systems 2010 Worldwide Gasification Database”. United States Department of Energy. Archived from the original on 1 March 2013. Retrieved March 3, 2013.
- Jump up^ “Rembrandt” (2 August 2012). “China’s Coal to Chemical Future” (Blog post by expert). The Oil Drum.Com. Retrieved3 March 2013.
- Jump up^ Ken Yin (27 February 2012). “China develops coal-to-olefins projects, which could lead to ethylene self-sufficiency”. ICIS Chemical Business. Retrieved 3 March 2013.
- Jump up^ Didi Kirsten Tatlow (5 February 2013). “Worse Than Poisoned Water: Dwindling Water, in China’s North” (Blog in edited newspaper). International Herald Tribune. Retrieved 3 March2013.
- Jump up^ “Spill in China Underlines Environmental Concerns”. The New York Times. 2 March 2013. Retrieved 3 March 2013.
- Jump up^ “NYMEX.com: Coal”. Archived from the original on 2008-03-12. Retrieved 16 January 2008.[dead link]
- Jump up^ “ICE: Coal Futures”. Archived from the original on 10 November 2006. Retrieved 16 January 2008.
- Jump up^ Coal News and Markets (Archive) Department of Energy – see Bloomberg for realtime prices
- Jump up^ “Coal Prices and Charts – Data from Quandl”. quandl.com.
- Jump up^ Toxic Air: The Case for Cleaning Up Coal-fired Power Plants. American Lung Association (March 2011) Archived26 January 2012 at the Wayback Machine.
- Jump up^ “Deadly power plants? Study Fuels Debate: Thousands of Early Deaths Tied To Emissions.” MSNBC (2004-09-06) Retrieved 5 November 2008
- Jump up^ “The Unpaid Health Bill – How coal power plants make us sick”. Health and Environment Alliance. Retrieved 7 March2013.
- Jump up^ http://www.who.int/ipcs/features/air_pollution.pdf,
- Jump up^http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf,
- Jump up^ “WHO – Ambient (outdoor) air quality and health”. who.int. Retrieved 7 January 2016.
- Jump up^ “Black Lung Disease-Topic Overview”. WebMD.
- Jump up^ “Black Lung”. umwa.org. Retrieved 7 January 2016.
- Jump up^ “Coal”. epa.gov. Archived from the original on 2015-07-20.
- Jump up^ “Coal Ash: Toxic – and Leaking”. psr.org.
- Jump up^ Mara Hvistendahl. “Coal Ash Is More Radioactive than Nuclear Waste”. scientificamerican.com.
- Jump up^ “Annual Energy Outlook 2015”. United States Energy Information Administration. Retrieved 11 December 2015.
- Jump up^ World Coal Association “Environmental impact of Coal Use”
- Jump up^http://noharm.org/lib/downloads/climate/Coal_Literature_Review_2.pdf
- Jump up^ http://www.rmi.org/RFGraph-health_effects_from_US_power_plant_emissions
- Jump up^ Environmental impacts of coal power: air pollution. Union of Concerned Scientists
- Jump up^ R. K. Tiwary (2001). “Environmental Impact of Coal Mining on Water Regime and Its Management”. Water, Air, & Soil Pollution132: 185–199. doi:10.1023/a:1012083519667.
- Jump up^ L.A. Barrie; R.M. Hoff (1984). “The oxidation rate and residence time of sulphur dioxide in the arctic atmosphere”.Atmospheric Environment 18 (12): 2711–2722. doi:10.1016/0004-6981(84)90337-8.
- Jump up^ Human Impacts on Atmospheric Chemistry, by PJ Crutzen and J Lelieveld, Annual Review of Earth and Planetary Sciences, Vol. 29: 17 -45 (Volume publication date May 2001)
- Jump up^ Direct Testimony of James E. Hansen. State of Iowa
- Jump up^ “World Of Coke: Coke is a High Temperature Fuel”.www.ustimes.com. Retrieved 2016-01-16.
- Jump up^ “International Energy Annual 2006”. Energy Information Administration. 2008. Archived from the original on 23 May 2011., see data tables
- Jump up^ “International energy statistics”. U.S. Energy Information Administration. Retrieved 10 March 2014., see table
- Jump up^ “How much carbon dioxide is produced when different fuels are burned?”. eia.gov. Retrieved 7 January 2016.
- Jump up^ “COP21: New research points to falling carbon dioxide emissions”. Financial Times. Retrieved 7 January 2016.
- Jump up^ Nuwer, Rachel (2012-08-17). A 20-Year Low in U.S. Carbon Emissions. blogs.nytimes.com
- Jump up^ “Leave coal in the ground to avoid climate catastrophe, UN tells industry”.
- Jump up^ “Coal vs. Wind”. Union of Concerned Scientists. Retrieved2008-12-30.
- Jump up^ “Air Trends”. Environmental Protection Agency.
- Jump up^ “The Future of Coal”. Massachusetts Institute of Technology. Retrieved 2008-12-23.
- Jump up^ “Mercury and Air Toxics Standards (MATS)”. U.S. Environmental Protection Agency. Retrieved 2014-07-21.
- Jump up^ Pearce, Fred (2008-10-30). “Time to bury the ‘clean coal’ myth”. London: The Guardian. Retrieved 2008-12-23.
- Jump up^ “The True Cost of Coal” (PDF). Greenpeace. Retrieved2008-12-23.
- Jump up^ “Carbon Capture and Storage”. University of Edinburgh, School of Geosciences. Retrieved 2008-12-23.
- Jump up^ “Carbon Capture Plans get Reality Check”. Discovery Channel. Retrieved 2008-12-23.
- Jump up^ “Pre-combustion Carbon Capture Research”. Energy.gov. Office of Fossil Energy, U.S. Department of Energy. Retrieved22 July 2014.
- Jump up^ “Picking a Winner in Clean-Coal Technology”.
- Jump up^ “Post-combustion Carbon Capture Research”. Energy.gov. Office of Fossil Energy, U.S. Department of Energy.
- Jump up^ “R&D Facts – Oxy-Fuel Combustion” (PDF). National Energy Technology Laboratory, U.S. Department of Energy. Retrieved22 July 2014.
- Jump up^ “IGCC Project Examples – Kemper County IGCC Project”.Gasifipedia. National Energy Technology Laboratory, U.S. Department of Energy. Retrieved 22 July 2014.
- Jump up^ “Boundary Dam Integrated Carbon Capture and Sequestration Demonstration Project”. Global CCS Institute. Retrieved22 July 2014.
- Jump up^ “Vattenfall’s Project on CSS”. Vattenfall. Archived from the original on 26 October 2010.
- ^ Jump up to:a b http://discovermagazine.com/2009/feb/25-can-clean-coal-actually-work/?searchterm=clean%20coal “Can Clean Coal Actually Work?” article in Feb. 2009 issue, page 18, Retrieved 2009-05-11
- Jump up^ J. A. Campbell; D. L. Stewart; M. McCulloch; R. B. Lucke; R. M. Bean. “BIODEGRADATION OF COAL-RELAED MODEL COMPOUNDS” (PDF). Pacific Northwest Laboratory: 514–521.
- Jump up^ M.C. Potter (May 1908). “Bateria as agents in the oxidation of amorphous carbon”. Proceedings of the Royal Society of London Series B, Containing Papers of a Biological Character 80: 239–259.
- Jump up^ Peckham, Jack (2002). “Diesel Fuel News: Ultra-clean fuels from coal liquefaction: China about to launch big projects – Brief Article”. Diesel Fuel News. Retrieved 9 September2005.[dead link]
- Jump up^ Tingting, Si; Jing, Li (12 December 2008). “Coal-to-liquids project rescheduled to launch in early 2009”. China Daily. Retrieved 12 December 2008.
- Jump up^ “Sasol, Shenhua Group May Complete Coal-to-Fuel Plant by 2013”. Bloomberg. 7 January 2009. Retrieved 10 January2009.[dead link]
- Jump up^ “China”. United States Energy Information Administration.
- Jump up^ Elegant, Simon; Zhangjiachang (2 March 2007). “Where The Coal Is Stained With Blood”. TIME. Retrieved 25 July 2014.
- Jump up^ “The human cost of coal in the UK: 1600 deaths a year”. New Scientist.
- Jump up^ “Environmentalism”. The Economist. 4 February 2014. Retrieved 7 January 2016.
- Jump up^ Fisher, Juliya (2003). “Energy Density of Coal”. The Physics Factbook. Retrieved 25 August 2006.
- Jump up^ “How much coal is required to run a 100-watt light bulb 24 hours a day for a year?”. Howstuffworks. Retrieved 25 August2006.
- Jump up^ Analysis: Efficiency of coal-fired power stations – evolution and prospects. EurActiv. Retrieved on 24 August 2012.
- Jump up^ CO2 Carbon Dioxide Emissions from the Generation of Electric Power in the United States, DOE, EPA, 1999. Eia.doe.gov. Retrieved on 24 August 2012.
- Jump up^ “Sino German Coal fire project”. Retrieved 9 September2005.
- Jump up^ “Committee on Resources-Index”. Archived from the original on 25 August 2005. Retrieved 9 September 2005.
- Jump up^ “Snapshots 2003” (PDF). fire.blm.gov. Archived from the original (PDF) on 2006-02-18. Retrieved 9 September 2005.
- Jump up^ “EHP 110-5, 2002: Forum”. Archived from the original on 31 July 2005. Retrieved 9 September 2005.
- Jump up^ “Overview about ITC’s activities in China”. Archived from the original on 16 June 2005. Retrieved 9 September2005.[dead link]
- Jump up^ “Fire in The Hole”. Retrieved 5 June 2011.
- Jump up^ “North Dakota’s Clinker”. Retrieved 9 September 2005.
- Jump up^ “BLM-Environmental Education- The High Plains”. Archived from the original on 12 March 2005. Retrieved 9 September2005.[dead link]
- Jump up^ Lyman, Robert M.; Volkmer, John E. (March 2001).“Pyrophoricity (spontaneous combustion) of Powder River Basin coals–: Considerations for coalbed methane development”(PDF). Archived from the original (PDF) on 12 September 2005. Retrieved 9 September 2005.[dead link]
- Jump up^ “EIA projects little change in U.S. coal production in 2013”. U.S. Energy Information Agency. 3 December 2012. Retrieved20 December 2012.
- Jump up^ Lipton, Eric (19 December 2012). “Power Company Loses Some of Its Appetite for Coal”. The New York Times. Retrieved20 December 2012.
- Jump up^ Le, Phuong (March 25, 2013). “NW governors ask White House to exam coal exports”. The San Francisco Chronicle. Associated Press. Archived from the original on 26 March 2013. Retrieved March 26, 2013.
- Jump up^ Clifford Krauss (June 14, 2013). “Coal Industry Pins Hopes on Exports as U.S. Market Shrinks”. The New York Times. Retrieved June 15, 2013.
- Jump up^ Suhr, Jim (May 1, 2013). “Report: Ill. coal enjoyed record exports in 2012”. San Francisco Chronicle. Associated Press. Archived from the original on 2 May 2013. Retrieved May 2,2013.
- Jump up^ “EIA International Energy Statistics : Coal : Consumption”. Retrieved 10 June 2009.
- Jump up^ World Energy Council – World Energy Resources: 2013 Survey. (PDF) . Retrieved on 26 December 2015.
- Jump up^ Sherwood, Alan and Phillips, Jock. Coal and coal mining – Coal resources, Te Ara – the Encyclopedia of New Zealand, updated 2009-03-02
- Jump up^ EIA International Energy Annual – Total Coal Consumption (Thousand Short Tons). Eia.gov. Retrieved on 2013-05-11.
- Jump up^ Table 114. World Metallurgical Coal Flows By Importing Regions and Exporting Countries 1,2/ (million short tons). eia.doe.gov
- Jump up^ World Coal Flows by Importing and Exporting Regions. Eia.doe.gov. Retrieved on 24 August 2012.
- Jump up^ EIA International Energy Annual: Coal Overview 2010. Eia.gov. Retrieved on 24 August 2012.
- Jump up^ “Kentucky: Secretary of State – State Mineral”. 20 October 2009. Archived from the original on 27 May 2011. Retrieved7 August 2011.
- Jump up^ “Utah State Rock – Coal”. Pioneer: Utah’s Online Library. Utah State Library Division. Retrieved 7 August 2011.